
2023-02-22 13:26:05来源:医药魔方浏览量:370
国内外指南推荐的胃癌一线治疗方案
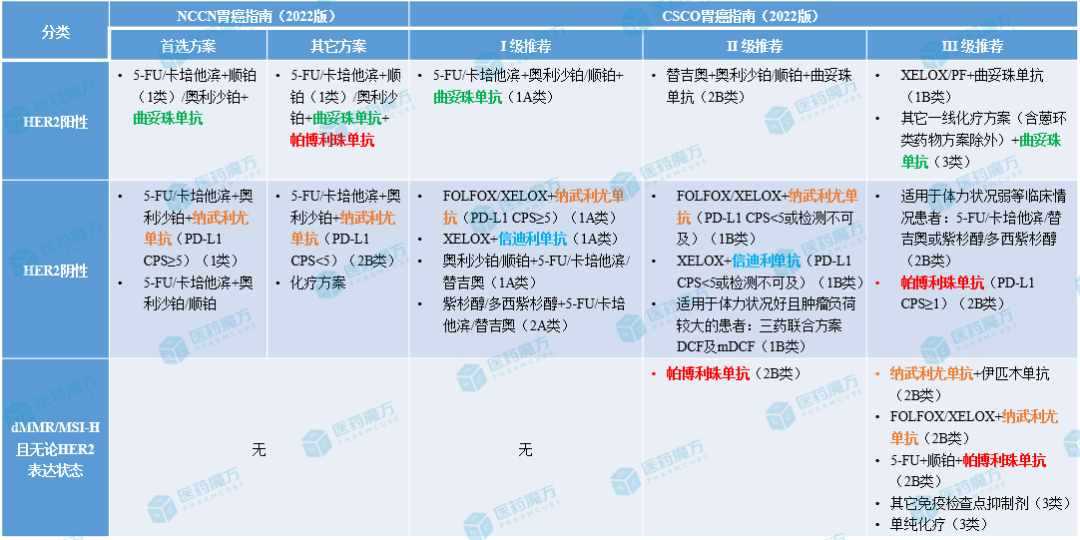
注:FOLFOX为亚叶酸钙+卡培他滨/5-FU+奥利沙铂,XELOX为奥利沙铂+卡培他滨,PF为顺铂+5-FU,DCF为甲地孕酮+5-FU+顺铂+多西紫杉醇,mDCF为5-FU+顺铂+多西紫杉醇
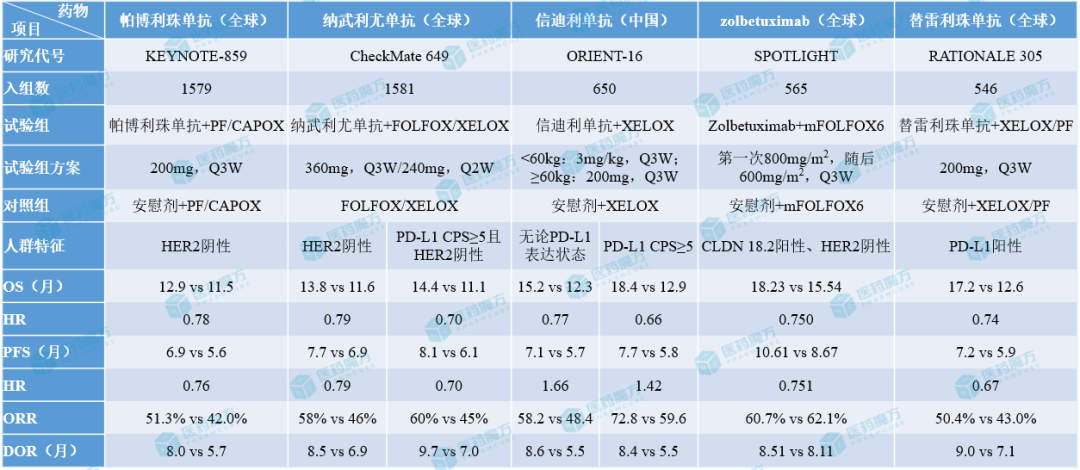
HER2阴性晚期胃癌一线治疗药物III期数据
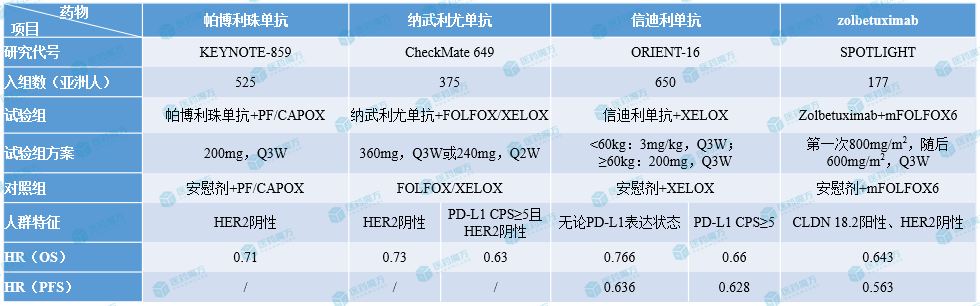
HER2阴性晚期胃癌一线治疗药物亚洲人群疗效
[1] Zolbetuximab + mFOLFOX6 as first-line (1L) treatment for patients (pts) with claudin-18.2+ (CLDN18.2+) / HER2− locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Primary results from phase 3 SPOTLIGHT study. ASCO GI 2023.
[2] Claudin-18 gene structure, regulation, and expression is evolutionary conserved in mammals. Gene. 2011;481(2):83-92.
[3] FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32(5):609-619.
[4] Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2019;49(9):870-876.
[5] Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. 2022;28(6):1189-1198.
[6] Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14(23):7624-7634.
[7] Association of CLDN18 Protein Expression with Clinicopathological Features and Prognosis in Advanced Gastric and Gastroesophageal Junction Adenocarcinomas. J Pers Med. 2021;11(11):1095. Published 2021 Oct 26.
[8] Evaluation and reflection on claudin 18.2 targeting therapy in advanced gastric cancer. Chin J Cancer Res. 2020;32(2):263-270.
[9] Safety, tolerability, and efficacy of the first-in-class antibody IMAB362 targeting claudin 18.2 in patients with metastatic gastroesophageal adenocarcinomas. Journal of Clinical Oncology. 2013.
[10] DOI: 10.1200/JCO.2016.34.18_suppl.LBA4001 Journal of Clinical Oncology 34, no. 18_suppl.
[11] Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249.
[12] https://gco.iarc.fr/today/home. Cancer fact sheets. Digestive organs. Stomach(C16).
[13] AJCC TNM分期8.0版胃癌.
[14] Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: results from a large Caucasian cohort study. Virchows Arch. 2019;475(5):563-571.
[15] HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma:Guideline From the College of American Pathologists,American Society for Clinical Pathology,and the American Society of Clinical Oncology.J Clin Oncol.2017 Feb;35(4):446-464.
[16] Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6(10):1571-1580.
[17] Nivolumab (NIVO) plus chemotherapy (chemo) vs chemo as first-line (1L) treatment for
advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma
(GC/GEJC/EAC): 3-year follow-up from CheckMate 649. ASCO GI 2023.
[18] 《NCCN临床实践指南:胃癌2022版》.
[19] 《CSCO胃癌诊疗指南2022版》.
[20] 默沙东ESMO Virtual Plenary新闻稿.
[21] LBA53 Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): First results of a randomized, double-blind, phase III study.ESMO 2021(32).
[22] Rationale 305: Phase 3 study of tislelizumab plus chemotherapy vs placebo plus chemotherapy as first-line treatment (1L) of advanced gastric or gastroesophageal junction
adenocarcinoma (GC/GEJC). ASCO GI 2023.
[23] Claudin18.2和PD-L1在中国胃腺癌和食管胃结合部腺癌中的表达. SITC 37th Annual Meeting 2022.
[24] https://clinicaltrials.gov/ct2/show/NCT03505320.
[25] Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J Clin Oncol. 2021;39(9):966-977.
声明:本文系药方舟转载内容,版权归原作者所有,转载目的在于传递更多信息,并不代表本平台观点。如涉及作品内容、版权和其它问题,请与本网站留言联系,我们将在第一时间删除内容