
2025-10-24 09:29:52来源:鼎泰集团TriApex浏览量:358
Guided by the 3R principle, evolved modern research is becoming more precise, predictive, and humane.
全文共:10352 字 3 图
预计阅读时长:25 分钟
2024 年 10 月 7 日, 美国 FDA 科学委员会(Advisory committees)召开的网络会议报告了来自新方法工作委员会(New Alternative Methods subcommittee)的最新进展,并发布了主题报告《Potential Approaches to Drive Future Integration of New Alternative Methods for Regulatory Decision-Making》。鼎泰团队在往期文章《Lang 来了!FDA 关于监管决策中 NAMs 使用的最新举措》中对这一次会议的主要内容进行了汇总。
点击图片跳转至原文
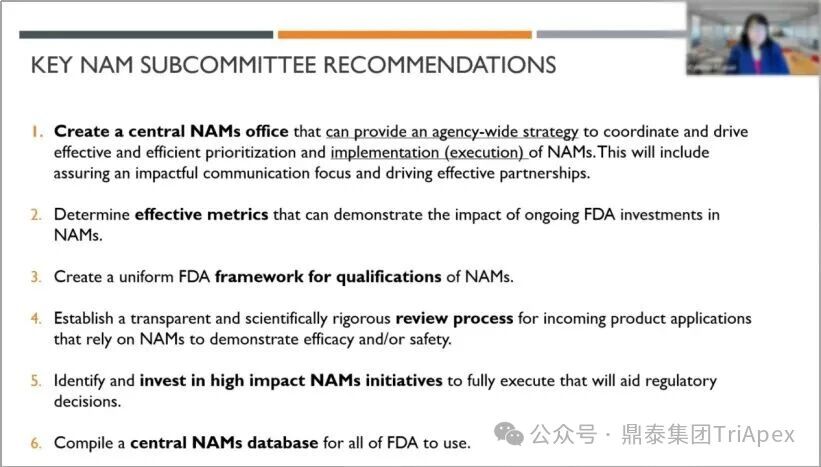
为推动新方法(New Alternative Methods, NAMs;最终中文术语以监管机构或行业共识为准)在科学评估和监管决策中的应用,该报告提出了 6 条建议:
建立 NAMs中心办公室(central NAMs office)
确定有效的评价指标(effective metrics)以衡量 FDA 对 NAMs 持续投资的影响
为NAMs制定统一的资格认定框架(framework for qualifications)
建立透明且科学严谨的审查流程,用于审查依靠 NAMs 来证明有效性和/或安全性的注册申请
识别并发展有助于监管决策的高影响力的关键项目,以全面执行并帮助监管决策
编制一个可供 FDA 所有部门使用的中央 NAMs 数据库(NAMs database)
时隔一年,承诺如期兑现。2025年10月8日, FDA 药品审评与研究中心(CDER)发布了一份详尽的立场文件,系统阐述了在药物非临床研究中使用 NAMs 和减少动物试验的具体情境与科学考量,并附有相关参考文献。该文件的发布,不仅是对去年所预告的监管改革方向的具体落实,更标志着药物非临床评估范式正在发生根本性转变 —— 从传统模式加速转化为更科学、高效、精准且基于风险的评估体系。
鼎泰团队始终专注科学评估,紧密关注全球监管政策的前沿动态。本期内容旨在对该文件进行深度学习和解读,以准确理解 FDA 在推动 NAMs 应用与动物试验优化方面的具体举措,进而切实提高在新范式下药物非临床研究的科学价值和对支持研发决策的重要作用。

历史背景
从“3R”理念到监管实践的演进
回顾历史,动物试验为保障人类用药安全做出了不可磨灭的贡献,但其局限性也日益凸显:种属差异导致的预测偏差、漫长的试验周期、高昂的成本以及日益增长的伦理关切,都促使科学界和监管机构寻求变革。
1959年,英国科学家William Russell和Rex Burch在其著作《The Principles of Humane Experimental Technique》中首次提出了以替代(Replacement)、减少(Reduction)和优化(Refinement)为核心的“3R”原则,为这场变革播下了思想的种子。然而,将这一伦理理念转化为广泛接受的行业实践和监管认同,却是一条漫漫长路。其核心挑战在于,如何证明非动物性替代方法在预测人体反应上与传统方法同等可靠,甚至更为精准?
过去十年,生物技术的突破性进展为解决这一挑战提供了可能。器官芯片、类器官、高内涵成像、人工智能驱动的计算机模型等 NAMs 技术如雨后春笋般涌现,它们能够更精细地模拟人体生理和病理状态,从而提供更具预测价值的数据。同时,监管层面的推动也随之而来。2022年,美国通过的《FDA现代化法案2.0》消除了对必须使用动物试验的强制性规定,为 NAMs 的应用扫清了法律障碍。
在此背景下,FDA CDER 作为全球药物监管的权威机构,其动向具有风向标意义。去年,我们曾在《Lang 来了!FDA 关于监管决策中 NAMs 使用的最新举措》一文中深入解读了 FDA 对 NAMs 的积极态度,并预示了 FDA 将更系统地在监管决策中整合这些新工具。如今,CDER 发布的这份最新文件,正是对这一承诺的兑现。它不再是 FDA 的理念宣导,而是有可能成为审评实践中具体应用的“操作手册”,标志着 NAMs 已从探索阶段进入常规审评的工具箱。
核心内容解析
指导药物非临床研究优化设计的研发情境
FDA CDER 以表格形式系统梳理了可接受的优化非临床研究设计的药物研发情境。这些情境涵盖减少样本量、减少动物种属数量以及使用 NAMs等多种策略,但不包括 505(b)(2) 途径的申请、仿制药和生物类似药,以及与杂质和辅料相关的研究。该文件以其详尽的表格为核心,为申请人在各类非临床研究中如何减少动物使用和优化试验设计提供了清晰的指引,以下是几个关键领域的深度解读:
(一) 安全药理学的“体外革命”
在传统研发中,评估药物对心血管系统(如 QT 间期延长风险)或中枢神经系统的影响,通常需要在特定的动物模型上进行。文件明确指出,体外试验系统(如细胞培养、离子通道、酶和受体) 完全可以作为可靠的试验系统。值得关注的是,对于致心律失常风险,一个经过适当验证的计算机或体外预测模型,可以根据其特定的使用情境,直接用于评估人类发生尖端扭转型室性心动过速的风险。这颠覆了以往主要依赖动物试验的范式,将安全性评估的关口前移到了更基础的分子和细胞水平。以下基于 FDA 原文翻译和理解对不同安全药理学研究情境下的监管预期和机会进行了分析:
情境 1:General safety pharmacology testing 一般安全药理学试验
Safety pharmacology endpoints may be incorporated into general toxicity studies in order to reduce animal use. This practice is routine for biologics. May include non-animal methods. May be based on a risk assessment that includes information from biodistribution data for radiopharmaceuticals.
安全药理学终点可纳入一般毒理研究中,以减少动物使用。这一做法在生物制品研究中已属常规。可包含非动物方法。
对于放射性药物,可基于包含其生物分布数据的风险评估。
情境 2:Cardiovascular safety pharmacology studies 心血管安全药理学研究
In vitro preparations can be used as test systems (e.g., cell cultures, receptors, ion channels, transporters and enzymes). In vitro systems can be used in supportive studies (e.g., to obtain a profile of the activity of the substance or to investigate the mechanism of effects observed in vivo).
An appropriately qualified proarrhythmia risk prediction model could be used according to its context of use to assess the possibility of torsades de pointes (TdP) in humans.
The analysis of QTc interval together with adequate pharmacokinetic sampling makes it possible to perform dedicated exposure-response modeling similar to concentration-QT analysis for clinical QT studies.
Different models, including in silico, in vitro, ex vivo and in vivo models, have the potential to be used as part of an integrated risk assessment strategy to evaluate the proarrhythmic risk of QT-prolonging pharmaceuticals in humans.
体外试验系统(例如,细胞培养物、受体、离子通道、转运体和酶)可用作试验系统使用。体外系统可用于支持性研究(例如,获取物质活性谱或探索体内观察到的效应机制)。
可根据其使用背景,采用经过适当验证的致心律失常风险预测模型,评估人类发生尖端扭转型室性心动过速的可能性。
通过 QTc 间期分析与充分的药代动力学采样,可建立类似于临床 QT 研究中浓度 -QT 分析的专用暴露-反应模型。
包括计算机模拟、体外、离体和体内模型在内的不同模型,均有潜力作为评估延长 QT 间期药物在人类中致心律失常风险的综合风险评估策略的一部分。
情境 3:Treatments for severely debilitating or life-threatening diseases (i.e., advanced cancer, several hematologic disorders, and graft-versus-host disease) 治疗严重衰弱或危及生命的疾病(例如,晚期癌症、多种血液系统疾病及移植物抗宿主病)
Endpoints may be integrated into pivotal toxicology studies. In the absence of a specific risk for patients in clinical trials, such studies will not be called for to support clinical trials or for marketing.
终点可被整合至关键性毒理学研究中。若临床试验患者无特定风险,则无需此类研究来支持临床试验或上市申请。
情境 4:Microdose radiopharmaceutical diagnostic drugs 微剂量放射性诊断药物
Not warranted.
无需进行(安全药理学研究)。
情境 5:To evaluate seizure risk 评估癫痫发作风险
In vitro or in silico methods could hypothetically be developed and used to predict human-relevant seizure risk and effect on the central nervous system.
理论上可开发并使用体外或计算机模拟方法来预测人类相关的癫痫发作风险及对中枢神经系统的影响。
情境 6:To evaluate drug-induced liver injury 评估药物性肝损伤
In vitro liver models have been developed to predict hepatotoxicity and drug-induced liver injury by assessing changes in liver biomarkers and functional endpoints.
已开发出体外肝脏模型,通过评估肝脏生物标志物和功能性终点的变化来预测肝毒性及药物性肝损伤。
(二) 一般毒理研究的“简化之道”
在慢性毒理学研究中,文件鼓励采用“证据权重(WoE)”原则。例如,对于生物制品,如短期研究表明啮齿类和非啮齿类(如 NHP)在药理学上均相关且未显示差异,则鼓励使用系统发育水平更低的单一种属进行长期研究,而非默认进行两种种属的试验。此外,文件明确,对于微剂量放射性诊断药物,重复给药毒性研究“无需进行”;对于治疗严重疾病的药物,慢性毒性研究的持续时间可以缩短至三个月。这些基于风险的科学考量,在确保安全的前提下,大幅减少了动物的使用数量和痛苦。
情境 1:Acute toxicity studies for small molecules 小分子药物的急性毒性研究
When appropriately conducted dose-escalation studies or short-duration dose-ranging studies are available to inform on the acute toxicity risk for small molecules, stand-alone, single-dose acute toxicity studies are not warranted.
对于小分子药物,若已通过适当的剂量递增研究或短期剂量范围确定试验明确了急性毒性风险,则无需再开展单独的单次给药急性毒性研究。
情境 2:Chronic toxicology studies for small molecule drugs (6-9 months; rodent and non-rodent, respectively) 小分子药物的慢性毒性研究(6-9个月,啮齿类和非啮齿类)
Toxicokinetic and pharmacokinetic analyses may be conducted during general toxicity studies.
毒代动力学和药代动力学分析可在一般毒理研究中进行。
情境 3:Chronic toxicology studies for biologics 生物制品的慢性毒性研究
If both rodents and non-rodents (nonhuman primates or NHPs) are pharmacologically relevant, use a single species if no differences are noted in short-term studies. Use the phylogenetically lower species. Chronic toxicology studies can be six months long.
Information on recovery can be obtained by an understanding that the particular effect observed is generally reversible/nonreversible; thus, inclusion of recovery animals is not necessarily warranted. When warranted, they only need to be included in one toxicology study.
若啮齿类和非啮齿类(NHP)均为相关种属,且在短期研究中未见差异,则可使用单一种属,并优先选择系统发育水平较低的种属。慢性毒性研究可设为 6 个月。
通过了解观察到的特定效应是可逆/不可逆来获取恢复性信息;因此不一定需要设置恢复期动物。若确有必要,仅需在一项毒理学研究中设置即可。
情境 4:Microdose radiopharmaceutical diagnostic drugs 微剂量放射性诊断药物
Repeat-dose toxicity is not warranted.
无需开展重复给药毒性研究。
情境 5:Treatments of severely debilitating or life-threatening diseases (i.e.,several hematologic disorders, advanced cancer, graft-versus-host disease) 治疗严重衰弱或危及生命的疾病(如多种血液系统疾病、晚期癌症、移植物抗宿主病)
Small molecule drugs and biologics: chronic toxicity is of three-month duration. When the investigational pharmaceutical extends survival or lessens the severity or the frequency of a debilitating event, toxicology studies of six to nine months duration are generally not warranted.
For an individualized antisense oligonucleotide, a single three-month toxicity study is considered adequate to assess safety for initiating human dosing, dose escalation, and chronic treatment.
对于小分子药物与生物制品:慢性毒性研究可设为 3 个月。若研究药物能延长生存期或减轻衰弱性事件的严重程度或发生频率,则通常无需进行为期 6 至 9 个月的毒理学研究。
对于个体化反义寡核苷酸药物,单项为期 3 个月的毒性研究足以评估人体给药、剂量递增和长期治疗的安全性。
情境 6:Orally inhaled nicotine products (for use as medical product, [e.g., smoking cessation]) 口服吸入尼古丁产品(用作医疗产品,如戒烟)
If the exposure is within range of exposure from lawfully marketed tobacco products or an approved product, no new nonclinical toxicity data is required.
若暴露量处于合法上市烟草产品或已批准产品的暴露范围内,则无需新的非临床毒性数据。
情境 7:General toxicity for a combination of two or more previously marketed drugs or biologics 两种或多种已上市药物联合用药的一般毒理研究
A bridging study may be appropriate, provided the duration is sufficient to elicit the toxicity of concern. For example, a three-month general toxicity bridging study could be considered for a chronic indication. If the two products have sufficient nonclinical and clinical characterization, combination animal studies may not be warranted.
若桥接试验研究周期内足以暴露所关注的毒性反应,则可采用此类研究设计。例如,对于慢性适应症,可考虑进行为期 3 个月的一般毒理桥接研究。若两种产品已完成充分的非临床和临床表征,则可能无需进行联合用药动物研究。
情境 8:General toxicity for a combination of two or more previously marketed drugs or biologics 两种或多种已上市药物联合用药的一般毒理研究(用于晚期癌症)
In general, toxicology studies investigating the safety of combinations of pharmaceuticals intended to treat patients with advanced cancer are not warranted.
If sufficient clinical data (e.g., a completed phase 1 or a monotherapy phase within phase 1) are available with the individual pharmaceuticals, additional nonclinical toxicology data may not be warranted. A rationale to support the combination should be provided, which can include in vitro or in vivo pharmacology data or a literature assessment.
If there is no or very limited human safety data for one of the combination components, a nonclinical pharmacology study of the combination should be considered, in addition to the toxicology studies with the single agents.
For pharmaceuticals that are pharmacologically inactive in animal species, assessment of combination can be based on relevant in vitro tests and/or a mechanistic understanding of target biology.
若联合用药拟用于治疗晚期癌症患者,通常无需开展联合用药的毒理学研究。
若各单药已具备充分的临床数据(如已完成的 I 期研究或 I 期内的单药治疗阶段),则可能无需额外的非临床毒理学数据。但应提供支持该组合的理由,可包括体外或体内药理学数据或文献资料。
若某一单药缺乏或仅有极有限的人体安全性数据,除单药毒理学研究外,还应考虑进行该组合的非临床药理学研究。
对于在动物种属中无药理学活性的药物,可基于相关体外试验和/或对靶点生物学的机制理解进行组合评估。
(三) 致癌性评估的“范式转移”
传统的长期动物致癌性研究耗时两年,需要使用大量动物。文件提出了根本性的转变。
首先,对于许多药物(如内源性物质替代疗法、明确遗传毒性的药物、短期使用药物),致癌性研究可能完全不需要。其次,对于需要评估的情况,大鼠的两年研究可以被 WoE 风险评估所替代,而小鼠的两年致癌性试验则可以用转基因小鼠的六个月研究来替代,后者使用的动物数量更少。对于生物制品,文件更是明确指出,标准致癌性生物测定“通常不适用”,转而推荐基于靶点生物学、疾病模型等所有可用信息的产品特异性评估。这要求研发者从“机械地完成试验清单”转向“深度理解药物作用机制”。
情境 1:Carcinogenicity studies are not always needed 致癌性研究并非总是必需
Carcinogenicity studies are not generally needed for endogenous substances given as replacement therapy (i.e., physiological levels).
When a drug is unequivocally genotoxic, a carcinogenicity study is not warranted as the drug is presumed to be trans-species carcinogen.
Pharmaceuticals administered infrequently or for short duration of exposure (e.g., anesthetics and radiolabeled imaging agents) do not need carcinogenicity studies unless there is cause for concern.
对于作为替代疗法(即生理水平)给予的内源性物质,通常无需致癌性研究。
当药物明确具有遗传毒性时,无需进行致癌性研究,因为该药物被推定为跨种属致癌物。
对于给药频率低或暴露时间短的药物(例如,麻醉剂和放射性标记成像剂),除非存在特定关注点,否则无需进行致癌性研究。
情境 2:General replacement of two long-term carcinogenicity studies for pharmaceuticals 药物两项长期致癌性研究的一般替代方案
The two-year study in rats may be substituted with a weight of evidence (WoE) risk assessment. A WoE assessment may inform whether a two-year rat study is needed.
An alternative to a two-year mouse study may be a six-month study in transgenic strains of mice, which typically employ fewer animals.
大鼠的两年致癌性试验可用 WoE 风险评估替代。WoE 评估可为是否需要开展该研究提供依据。
小鼠两年致癌性试验的替代方案可以是转基因小鼠的六个月研究,后者通常使用的动物数量较少。
情境 3:Biologics 生物制品
Standard carcinogenicity bioassays are generally inappropriate. Product-specific assessment for carcinogenic potential is recommended. Use all available information (e.g., knock-out or animal disease models, human genetic diseases, class effects, and target biology).
标准致癌性试验通常不适用。建议针对具体产品进行致癌潜力评估。应利用所有可获得信息(例如,基因敲除或动物疾病模型、人类遗传疾病、类效应和靶点生物学)。
情境 4:Treatments of severely debilitating or life-threatening diseases (i.e., advanced cancer, certain hematologic disorders; graft-versus-host disease) 治疗严重衰弱或危及生命的疾病(例如,晚期癌症、多种血液系统疾病及移植物抗宿主病)
Animal carcinogenicity studies are generally not warranted when any of these applies: Carcinogenicity signals have been identified in general toxicology studies or in humans; the drug is a genotoxic cytotoxic agent; a WoE risk assessment was conducted that can determine the outcome; product-specific agreement (see the guidance for GnRH analogues in prostate cancer).
When an animal study is warranted, it could be conducted postapproval, as appropriate (e.g., when clinical development is short and carcinogenicity studies would delay product approval).
当出现以下任何情况时,通常无需进行动物致癌性研究:在一般毒理学研究或人体中已识别出致癌信号;该药物是遗传毒性细胞毒剂;已进行可确定结果的 WoE 风险评估;存在针对特定产品的共识(参见前列腺癌 GnRH 类似物相关指南)。
当确需进行动物研究时,可酌情在批准上市后进行(例如,当临床开发周期较短且致癌性研究会推迟产品批准时)。
情境 5:Rare diseases 罕见病
Carcinogenicity study results may be submitted with the marketing application. In certain circumstances, submission of these data may be deferred to after approval.
致癌性研究结果可与上市申请一同提交。在特定情况下,这些数据的提交可推迟至批准后。
(四) 生殖与发育毒性(DART)的“替代前沿”
CDER 积极鼓励使用体外、离体和非哺乳动物体内替代试验。文件指出,如果采用分层或组合的替代方法,其提供的用于人类安全保障的置信度应至少等同于当前的传统试验范式。对于某些特定药物(如基因毒性抗癌药),胚胎-胎仔发育研究甚至被视为“非必需”。当生物制品仅在 NHP 动物中具有活性时,一项增强的围产期发育研究(ePPND)是足够的,而非进行多项独立研究。
情境 1:General reduction of fertility, embryofetal development, and/or pre- and postnatal development studies 一般DART研究
Studies may be submitted at the time of marketing application in certain circumstances.
A number of alternative in vitro, ex vivo, and nonmammalian in vivo assays (alternative assays) have been developed to detect potential hazards to embryo-fetal development. The use of alternative assays for these purposes is encouraged.
If alternative approaches are used for embryofetal development studies, multiple alternative assays used within a tiered or battery approach should provide a level of confidence for human safety assurance at least equivalent to that provided by the current testing paradigms.
If in vivo studies are used, use of the same species and strain as that in already completed toxicity studies can eliminate the need to use additional animals or conduct additional studies.
Combination studies can be employed to assess all relevant stages of the reproductive process using fewer animals.
在某些情况下,研究可在上市申请时提交。
已开发出多种体外、离体和非哺乳动物体内试验(替代试验)用于检测胚胎-胎仔发育的潜在风险。鼓励采用此类替代试验。
若采用替代方法用于胚胎-胎仔发育研究,则在分级或组合方法使用的多种替代试验,应提供至少等同于当前试验范式所提供的人类安全保障置信度。
若开展体内试验,采用与已完成的毒性研究相同的种属和品系可避免使用额外的动物或开展额外研究。
可采用组合研究,使用更少的动物评估生殖过程的所有相关阶段。
情境 2:Treatments of severely debilitating or life-threatening diseases (i.e., advanced cancers, certain hematologic disorders, graft-versus-host disease) 治疗严重衰弱或危及生命的疾病(如晚期癌症、特定血液系统疾病、移植物抗宿主病)
Embryofetal developmental studies are not considered essential for pharmaceuticals that are genotoxic and target rapidly dividing cells or belong to a class that has been well characterized as causing developmental toxicity.
In cases where an embryofetal developmental toxicity study (including pilot non-GLP study) is positive for embryofetal lethality or teratogenicity, a confirmatory study in a second species is usually not warranted.
A WoE approach showing potential for reproductive toxicity may eliminate the need to conduct a dedicated study.
A WoE risk assessment can be used for small molecule drugs and biopharmaceuticals. The WoE can include a literature assessment of target biology, use of alternative assays such as fit-for-purpose in vitro or ex vivo, or nonmammalian in vivo assays.
对于具有遗传毒性并靶向快速分裂细胞或属于已充分表征可引起发育毒性的药物,认为胚胎-胎仔发育研究是非必需的。
若胚胎-胎仔发育毒性研究(包括预试验 non-GLP 研究)结果显示胚胎-胎仔致死性或致畸性呈阳性,通常无需在第二种属中进行确证性研究。
采用 WoE 证明存在生殖毒性潜在风险时,可免于开展专项研究。
WoE 风险评估可用于小分子药物和生物制品。WoE 可包括对靶点生物学的文献评估、适用的体外或离体替代试验,或非哺乳动物体内试验等。
情境 3:Biologics 生物制品
When the product is active in rodents and rabbits, sponsors should use these species (instead of NHPs) for EFD assessment. When a study is positive, a study in the second species is not warranted.
DART studies may not be warranted if the WoE risk assessment suggests an adverse effect on fertility or pregnancy.
If a biologic is only active in NHPs, conduct a single enhanced pre-/postnatal development study. Additionally, fertility can be assessed by evaluation of the reproductive organs in general toxicology studies rather than a standalone study.
If clinical studies include sufficient precautions to prevent pregnancy, studies may be conducted during phase 3. An alternative model can be used in place of NHPs if appropriate scientific justification is provided.
当产品在啮齿类和兔中具有活性时,研发者应使用这些种属(而非 NHP)进行胚胎-胎仔发育评估。当一项研究结果呈阳性时,无需再在第二种属中进行研究。
若 WoE 风险评估表明对生育力或妊娠存在不良影响,则可能无需进行 DART 研究。
若某生物制品仅在 NHP 中具有活性,则需进行一项 ePPND 研究。此外,可通过评估一般毒理学研究中的生殖器官来评估生育力,无需进行独立研究。
若临床研究已采取充分的避孕措施,则相关研究可在 III 期临床期间进行。若提供适当的科学依据,可使用替代模型替代 NHP。
情境 4:Oligonucleotides 寡核苷酸
There can be cases where the WoE from existing data may be considered sufficient to communicate the risk to reproduction and embryofetal development, and no additional nonclinical studies are warranted.
某些情况下,现有数据的 WoE 可能足以说明其对生殖和胚胎-胎仔发育的风险,而无需开展额外的非临床研究。
情境 5:Microdose radiopharmaceutical diagnostic drugs/ Therapeutic radiopharmaceuticals 微剂量放射性诊断药物/治疗性放射药物
Not warranted
无需进行 DART 研究。
(五) “证据权重”成为贯穿始终的核心理念
通览全文,WoE 风险评估犹如一条金线,贯穿于所有决策之中。它代表了一种思维模式的转变:从依赖单一、规定性的动物研究,转向综合所有相关数据(包括化学结构、靶点生物学、体外数据、早期临床数据等)进行综合判断。无论是豁免幼龄动物研究,还是替代长期的慢性毒性或致癌性研究,WoE 都是实现科学、灵活监管决策的关键工具。
情境 1(致癌性研究):General replacement of two long-term carcinogenicity studies for pharmaceuticals 药物两项长期致癌性研究的一般替代方案
The two-year study in rats may be substituted with a weight of evidence (WoE) risk assessment. A WoE assessment may inform whether a two-year rat study is needed.
大鼠的两年致癌性试验可用 WoE 风险评估替代。WoE 评估可为是否需要开展该研究提供依据。
情境 2(致癌性研究):Treatments of severely debilitating or life-threatening diseases (i.e., advanced cancer, certain hematologic disorders; graft-versus-host disease) 治疗严重衰弱或危及生命的疾病(例如,晚期癌症、多种血液系统疾病及移植物抗宿主病)
Animal carcinogenicity studies are generally not warranted when any of these applies: Carcinogenicity signals have been identified in general toxicology studies or in humans; the drug is a genotoxic cytotoxic agent; a WoE risk assessment was conducted that can determine the outcome; product-specific agreement (see the guidance for GnRH analogues in prostate cancer).
当出现以下任何情况时,通常无需进行动物致癌性研究:在一般毒理学研究或人体中已识别出致癌信号;该药物是遗传毒性细胞毒剂;已进行可确定结果的 WoE 风险评估;存在针对产品的共识(参见前列腺癌 GnRH 类似物相关指南)。
情境 3(DART 研究):Treatments of severely debilitating or life-threatening diseases (i.e., advanced cancer, certain hematologic disorders; graft-versus-host disease) 治疗严重衰弱或危及生命的疾病(如晚期癌症、特定血液系统疾病、移植物抗宿主病)
A WoE approach showing potential for reproductive toxicity may eliminate the need to conduct a dedicated study.
A WoE risk assessment can be used for small molecule drugs and biopharmaceuticals. The WoE can include a literature assessment of target biology, use of alternative assays such as fit-for-purpose in vitro or ex vivo, or nonmammalian in vivo assays.
采用 WoE 证明存在生殖毒性潜在风险时,可免于开展专项研究。
WoE 风险评估可用于小分子药物和生物制品。WoE 可包括对靶点生物学的文献评估、适用的体外或离体替代试验,或非哺乳动物体内试验等。
情境 4(DART 研究):Biologics 生物制品
DART studies may not be warranted if the WoE risk assessment suggests an adverse effect on fertility or pregnancy.
若 WoE 风险评估表明对生育力或妊娠存在不良影响,则可能无需进行 DART 研究。
情境 5(DART 研究):Oligonucleotides 寡核苷酸
There can be cases where the WoE from existing data may be considered sufficient to communicate the risk to reproduction and embryofetal development, and no additional nonclinical studies are warranted.
某些情况下,现有数据的 WoE 可能足以说明其对生殖和胚胎-胎仔发育的风险,而无需开展额外的非临床研究。
情境 6(幼龄动物研究):General reduction of juvenile animal studies (JA ) 减少幼龄动物研究(JAS)的一般方案
WoE-based decisions should be made. JAS are not considered important to support short-term pharmacokinetic studies in pediatric populations, and a WoE-based decision should be made. Changing the design of a traditional nonclinical program can address pediatric concerns (e.g., dosing at a younger age in repeat-dose toxicity). If JAS are conducted, combine assessment of endpoints in the same subset of animals.
应基于 WoE 做出决策。幼龄动物研究通常不被认为对支持儿科人群短期药代动力学研究具有重要性,应基于 WoE 做出决策。如需开展 JAS,则对同一动物亚组中的终点进行整合评估。
情境 7(特殊毒性及其他类型的毒性筛选):Immunotoxicity studies免疫毒性研究
Immunotoxicity could be addressed through in vitro assays, a WoE assessment, or by adding endpoints into the design of general toxicity or proof-of-concept studies. Stand-alone animal immunotoxicity studies should only be conducted if the WoE from the general animal toxicology studies, knowledge of the target biology and/or pharmacokinetic/pharmacodynamic data suggest a risk that should be better characterized.
免疫毒性可通过体外试验、WoE 评估,或在一般毒理学或概念验证研究设计中增加相关终点来进行评估。仅当一般动物毒理学研究、靶点生物学知识和/或药代动力学/药效学数据的 WoE 提示存在需进一步明确的风险时,才应开展独立的动物免疫毒性研究。
情境 8(特殊毒性及其他类型的毒性筛选):Skin sensitization 皮肤致敏作用
The murine local lymph node assay (LLNA) is a validated sensitization assay that reduces the number of animals used and refines their treatment. CDER accepts negative LLNA data without further testing in the guinea pig test. The LLNA can also be used as part of a WoE evaluation to discriminate between strong and weak sensitizers.
小鼠局部淋巴结试验(LLNA)是一种经过验证的致敏试验,可减少所用动物数量并优化动物处理方式。CDER 接受阴性 LLNA 数据,无需在豚鼠试验中进行进一步研究。LLNA 也可作为 WoE 评估的一部分,用于区分强致敏物和弱致敏物。
情境 9(其他):Exaggerated pharmacology in oligonucleotide-based therapeutics 寡核苷酸类药物的放大药理学
If the effects of exaggerated pharmacology cannot be evaluated in toxicology studies, a literature-based WoE risk assessment of the potential adverse effects should be provided.
若放大药理学效应无法在毒理学研究中评估,则应提供基于文献的 WoE 风险评估,评估潜在的不良效应。
从概念到实践
NAMs 在监管决策中的实际应用
CDER 发布的上述文件的核心价值在于超越了原则探讨,提供了 NAMs 应用于监管决策的具体“监管案例”。这些附有详细参考文献的实例,不仅极具说服力,更标志着 NAMs 已从科学概念转化为审评实践中的具体工具。这些案例提示,NAMs 不再是未来的愿景,它们已经深度融入当下的监管科学,并实实在在地影响着药物的研发和审评进程。
情境 1:Drug efficacy for genetic variants of cystic fibrosis and Fabry disease(推测可能是 Migalastat)治疗囊性纤维化与法布里病
An in vitro approach was used to assess the functional and biochemical response of mutated or dysfunctional protein(s) in the presence of drug to make inferences about the potential for response in vivo. The findings from these data supported expanding the indications for the drugs to mutations not tested clinically.
通过体外方法评估 Migalastat 对特定基因突变蛋白的功能影响,FDA 批准了将 Migalastat 适应症扩大至未经临床验证的突变类型,为罕见病患者带来了福音。
情境 2:Drug safety of remdesivir 瑞德西韦安全性
In silico secondary pharmacology approaches were used to predict the potential renal safety risk of remdesivir based on its structure, physiochemical properties, and affinity for targets.
在评估其肾脏安全性时,采用了计算机模拟次要药理学方法,基于其结构、理化性质和靶点亲和力预测了潜在风险,减少了对额外动物试验的依赖。
情境 3:Drug efficacy for a new naloxone indication 纳洛酮新适应的疗效
The FDA’s independent modeling and simulation supports the sponsor’s claim that administration of the naloxone auto-injector (NAI) 10 mg resulted in a higher percentage of subjects recovering from respiratory depression for middle and high opioid doses compared to NAI 2 mg. In addition, the FDA’s independent modeling and simulation supported the sponsor’s second claim that administration of NAI 10 mg before fentanyl or carfentanil exposure can prevent rapid and profound opioid-induced respiratory depression.
FDA 的独立建模与模拟支持了申请人的申请事项,即施用 10 mg 纳洛酮自动注射器与 2 mg 纳洛酮自动注射器相比,在中高阿片类药物剂量下,能从呼吸抑制中恢复的受试者比例更高。此外,FDA 的独立建模与模拟也支持了申请人的第二项申请事项,即在芬太尼或卡芬太尼暴露前施用 10 mg 纳洛酮自动注射器可预防快速且严重的阿片类药物诱导的呼吸抑制。
情境 4:CD3 bispecifics CD3 双特异性抗体长周期毒理试验
A 2022 research project showed that for the CD3 bispecific products examined, absence of chronic (i.e., three-month) toxicology studies did not have adverse regulatory impact. When three-month studies were conducted, they were not informative; toxicities were predicted based on the available information (pharmacology and short-term toxicology, target biology, and phase 1 human data). Thus, a WoE assessment may predict the outcome of chronic dosing. Data collected (2017) shows common toxicities in animals from CD3 bispecifics (e.g., cytokine release syndrome and inflammatory response) and challenges associated with conducting three-month toxicology (e.g., anti-drug antibody formation, confounding the results).
2022 年的一项研究表明,CD3 双抗即使未开展 3 个月慢性毒理学研究,也未对其监管审评产生不利影响。当开展 3 个月研究时,并未提供更多有价值信息;其毒性特征完全可基于现有信息(药理学与短期毒理学数据、靶点生物学特性及Ⅰ期人体数据)进行预测。因此,WoE 评估或能预测长期给药的结果。2017 年的数据显示了 CD3 双抗在动物中的常见毒性(如细胞因子释放综合征和炎症反应),以及开展 3 个月毒理学研究面临的挑战(如抗药抗体形成干扰结果解读等)。
情境 5:Antibody-drug conjugates (ADCs) 抗体偶联药物
Data collected for ADCs with cytotoxic payloads indicate that toxicities of these products are mainly from the payload. Toxicity profiles of ADCs that contain the same payload were comparable in animals, independent of the monoclonal antibody moiety. Thus, streamlined approaches reducing use of NHPs may be applicable for certain ADCs. Studies of the linker or the monoclonal antibody alone are not warranted.
数据表明,含相同细胞毒性有效载荷的 ADC,其毒性特征在动物中具有可比性,与抗体部分无关。因此,对于某些 ADC,简化 NHP 动物使用的策略是可行的,无需再进行连接子或裸抗的研究。
情境 6:PD-(L)1 blocking antibodies PD-(L)1 阻断抗体
For these anti-cancer products, a WoE risk assessment may be considered in lieu of a three-month toxicology study.
此类抗癌产品,可以考虑采用 WoE 风险评估来替代为期 3 个月的毒理学研究。
结语
未来展望与行业启示
目前,动物模型在评估复杂生理系统时仍存在一定的局限性,在可预见的未来,NAMs 与经过验证的动物试验将构成一个迭代互促的协同体系。NAMs 用于早期高风险信号的筛查与机制探索,而动物模型在评估复杂系统毒性等方面仍具关键价值。监管决策的核心,正从单一研究转向对多元化证据体系的综合评估。
与此同时,NAMs 的广泛应用仍面临着多重挑战,如 AI 与类器官等技术的预测准确性、可靠性,以及对复杂毒性的评估能力,均需更多高质量数据的持续验证;此外,各国监管机构对 NAMs 的接受标准尚存在差异,这也可能影响其国际化应用的同步推进。
尽管如此,CDER 此次发布的文件,已向整个制药行业传递出清晰的信号:采用 NAMs 已不再是一个可选动作,而是未来药物研发体系中的核心能力。对于新药研发企业与 CRO 公司而言,这份文件也提供了以下启示:
1. 研发策略需前瞻布局
在项目立项与早期研究阶段,积极建设和利用与生物学相关性更高的体外模型及计算机预测平台,有助于在早期获得更具临床提示意义的数据,降低后续开发风险。
2. 与监管机构的沟通方式的转变
与监管部门的交流不应再局限于提供动物试验数据,而应逐步转变为基于 WoE 原则的总和评估,系统整合包括 NAMs 数据在内的多重证据,从而为研发决策构建更全面、扎实的科学依据。
3. 提升科学深度是必然要求
仅将动物试验视为“黑箱”检验的研发模式正在成为过去式。未来的药物开发更加依赖对疾病生物学、药物作用机制及靶点风险的深入理解,而 NAMs 正是实现这种科学洞察的有效工具。采用 NAMs 不仅是对动物福利与伦理诉求的积极响应,从科学性角度和研发回报角度看,它也具备显著优势 —— 有助于缩短研发周期、降低整体研发成本,并提高最终的成功率。
参考资料: